
SocraTec R&D provides services for clinical trials, specializing in pharmacokinetics and bioequivalence studies.
SocraTec R&D
SocraTec R&D is an expertise-driven leading full-service Contract Research Organisation (CRO) with more than 25 years of experience.
We operate our own state-of-the-art Clinical Pharmacology Units (CPUs) dedicated to clinical conduct of early phase trials in healthy subjects and patients.
Our team, based in 3 locations in Germany, is specialized on clinical research solutions in early and late phase drug development. With our Phase I CPUs and a strong international network, we provide the full range of clinical research services to the pharmaceutical, biotechnology, and medical device industries, including:
- Design Development, Scientific Advice & Expert Statements
- Clinical Trials Phase I-IV
- Quality Assurance
- Monitoring
- Data Management
- Biostatistics & Pharmacokinetics
- Medical Writing
- Pharmacovigilance
We manage and conduct clinical studies from the first application in humans, through proof-of-concept in the patient population, to efficacy studies in the late stages of clinical testing.
Your partner from bench to bedside … and beyond!
Focus topics
- Pharmacokinetics
- Bioavailability & Bioequivalence
- Drug-Drug Interactions
- Drug-Food Interactions
- Oncological Studies
- Ophthalmological Studies
- Paediatric Studies
- Gynaecological Studies
- Pain Studies
- Inhalatives
- Transdermal Therapeutics
- CNS & Sleep
- Hyperlipoproteinaemia
- Impaired renal function & dialysis
- Inflammatory bowel disease
- Pulmonary diseases
- Urology
location
Oberursel, Germany
Partner since
Founding year
Partner website



Our focuses:
Early Phase Studies
We are experts in bioavailability and bioequivalence studies across various dosage forms and absorption sites. Our clinical unit specializes in First-in-Human studies, including SAD, MAD, and food interaction studies. We routinely conduct pharmacodynamic and pharmacokinetic studies, including drug-drug interaction assessments. Additionally, we design and execute early-phase patient trials and integrated protocols, ensuring a comprehensive approach from study design to execution.
Phase II / IV Studies
With years of expertise in coordinating multicenter and multinational phase III and phase IV efficacy trials, as well as non-interventional studies such as PASS, DUS, and PAES, we bring a comprehensive approach to clinical research. As a full-service CRO, our support spans the entire process—from study design and site selection to monitoring, data management, statistical analysis, and medical writing. Our reach extends across Germany, Europe, the USA, Japan, and additional regions upon project-specific requests, ensuring global coverage tailored to your needs.
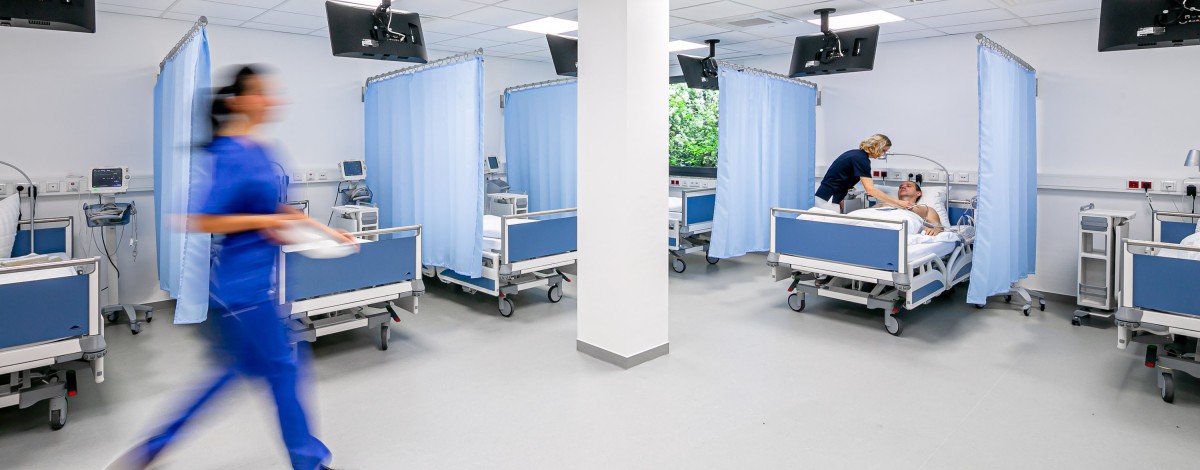
Main CPU
High throughput, high quality
- Total capacity: 54 beds
- Flexible layout on 1.400 m²
- Highly experienced clinical team
Main CPU
Located within maximum care hospital
- Total capacity: 6 beds
- Operated by the same experienced team
- Hospital supports with intensive care unit and anaesthesiology
Case Studies
Explore how we to drive innovation, enhance solutions, and achieve impactful results across industries. Discover our success stories.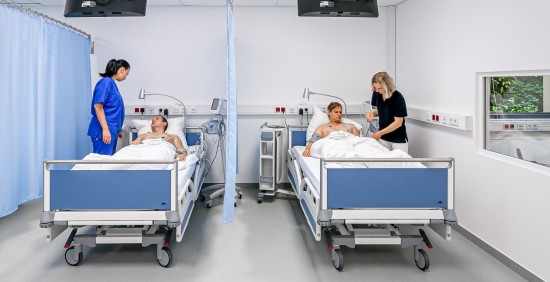
First-in-Human trials under highest safety standards in a thriving environment
An interview with our clinical pharmacology expert Dr. Ruwen Böhm. Read more in our latest Linkedin article here.
A list of published research projects can be found at https://socratec-pharma.de/library-publications
Certifications / accreditations:
The accreditation is only valid for the scope of accreditation listed in the customer documentation




